Following discussions with the team – notably Gabrielle Lebbink and Eleanor Velasquez – we began to open up a new thread around the possibilities of soil inoculation – that is – finding ways to move material from eco active parts of the existing forest to the new sites. This investigation is fuelled by/consistent with the need for the design of the artwork work to be of positive net benefit to the ecology of the emerging forest.
Gabrielle supplied some great information about ‘Re-wilding microbial/soil based communities’:
Contos P, Wood JL, Murphy NP, Gibb H. Rewilding with invertebrates and microbes to restore ecosystems: Present trends and future directions. Ecol Evol. 2021;11:7187–7200. https://doi.org/10.1002/ece3.7597. Downloaded y on [02/02/2024].
This article offered us some pointers of how we may be able to enhance soil biodiversity (potentially a part of an art/science process of trslocation of materials from the current forest to teh regeneration zone). I noted of that paper ..
Re-wilding of Microbe Communities (Notes, KMA 7/2/24)
Notes from: Contos P, Wood JL, Murphy NP, Gibb H. Rewilding with invertebrates and microbes to restore ecosystems: Present trends and future directions. Ecol Evol. 2021;11:7187–7200. https://doi.org/10.1002/ece3.7597. Downloaded from https://onlinelibrary.wiley.com/doi/10.1002/ece3.7597 by National Health and Medical Research Council, Wiley Online Library on [02/02/2024].
CONTEXT:
Invertebrates/microbes are key drivers of landscape scale ecosystem functions such as nutrient recycling and carbon sequestration, but many of these may still be functionally absent decades after a restoration process. The aim of any restoration project should actually be to reinstate ecosystem function and reestablishment of self-organising communities; and yet restoration ecologists rarely consider invertebrates and microbes which these make up the vast bulk of biodiversity and may drive key ecosystem recovery processes. Currently there is limited use of this approach in re-wilding projects – and often poor understanding of its importance.
Stepwise restoration – i.e. a species at a time – is becoming less realistic in a dynamic and rapidly changing world. Typical approaches to restoration involve plant only and plant and animal only approaches – which operate under what the authors call the ‘field of dreams paradigm’ – i.e. that if you build the habitat they will come! Such attempts often therefore ignore the ‘unseen majority’.
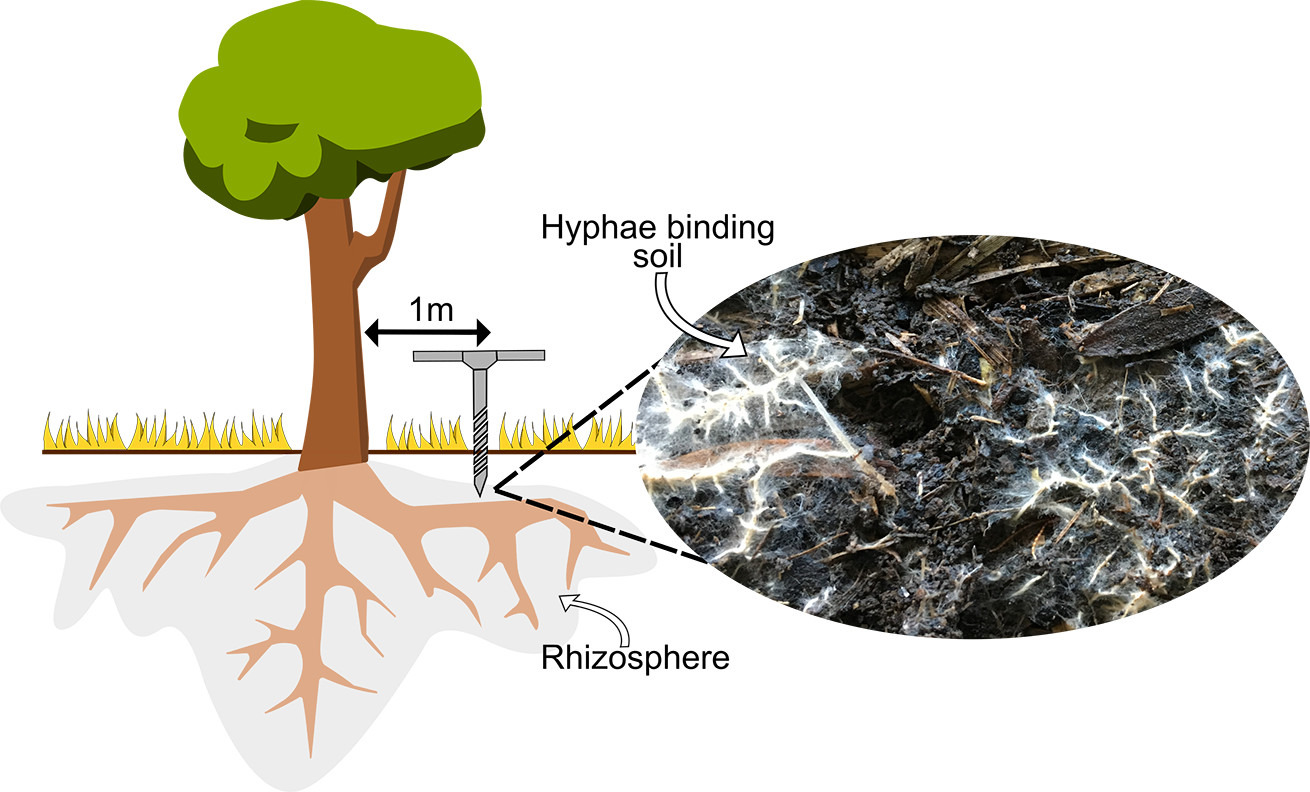
Normally microbial species translocations are done for the benefit of other species (i.e. to encourage back butterflies or for the benefit of a particular plant health)) rather than for themselves per se (e.g. introduced AMF fungi) – without considering whole communities. Furthermore, in most cases microbes are rarely monitored afterwards anyway. Hence some environments may remain functionally unrecovered 10 or 20 years later. NB The process of microbial re-wilding is more common in soil inoculation studies (soil transplants).
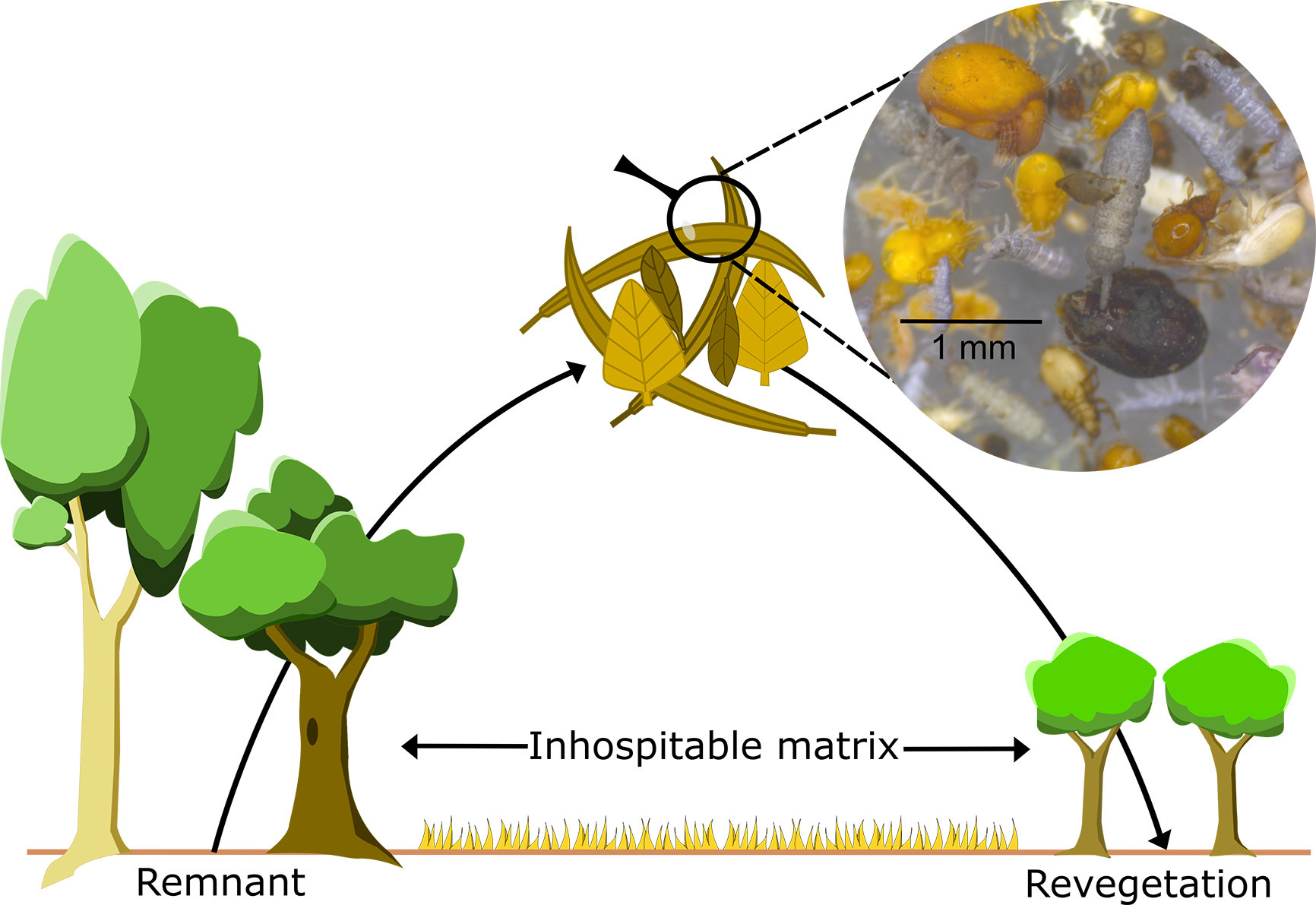
Whole of community microbial (and invertebrate?) rewilding is often practical due to ease of manipulation – e.g. in forest litter invertebrates and microbes (e.g. detritovores) are critical components – (see p7193). Whole of community rewilding typically involves transporting small subsets of whole habitats – normally from a nearby remnant site to a revegetation plot that maybe separated by an ‘inhospitable matrix’ – e.g. a pasture – and may include elements such as soil, dead wood and pond mud.
Its critical to avoid failures which will result in overall net loss of biota in an already stressed environment). The authors therefore suggest considering:
- suitability of renewed habitat to accommodate the microbes: The revegetation site should be ameliorated and receptive (physical and chemical limitations of soil) – and the source site very carefully scanned for invasives and pathogens.
- dispersal limitations of some invertebrates and microbes
- Distance of new site from existing vegetation ideally should not be far
- May work better in cool wet conditions when microbes and invertebrates are active and closer to surface of litter level (n.b. authors outside Australia)
Broadly speaking a project should
- Set restoration goals
- Evaluate the restoration trajectory
- Choose what to re-wild
- Conduct post re-wilding monitoring
- Evaluate success/failures.

See also this great podcast: https://www.futureecologies.net/listen/life-in-the-soil

These interesting learnings dovetailed with the research one of the other science academics (A/Prof Caroline Hauxwell) who is investigating diversity of fungal communities in soil, including Purpureocillium, which is a interesting fungus associated with pasture health and resilience to pests and diseases. She is interested in the impacts of burning on beneficial fungal symbionts (and thus the artwork site is an ideal choice) – something that ties in with her work on management of the pasture mealybug Heliococcus summervillei, which causes ‘pasture dieback’ (research that is co-funded with Meat and Livestock Australia). A/Prof Hauxwell is one of Australia’s foremost experts in this area and her research is extensive and widely recognised.
Dr. Hauxwell had already independently set up an experiment to monitor the artwork areas pre and post burn – so we were all ears to her process! Generously she invited Eleanor and I to join her class and get involved in some of her soil science – we jumped at this opportunity as an interesting creative opening.
To get more of a handle on this work – here are some excerpts from an email that A/Prof Caroline Hauxwell subsequently wrote – as part of a group email in Feb 2024.
We welcome, Keith Armstrong, who initiated the burn at SERF and who is very interested in this work. Welcome, Keith.
“For most of you the work really starts in week 3 with the field trip to collect soil samples at SERF.
The project work this year is a Deep Dive on Soil Endophytes. We’ll be focusing particularly on Purpureocillium lilacinum. The project builds on sampling that we did before and shortly after a burn site at SERF for capstone last year.
The student data identified changes in Purpureocillium abundance and distribution in the burned area.

We also know from our research that P. lilacinum isolates have significant morphological diversity, but ITS sequencing has shown only 1 sequence/variant. We hope to look a bit more closely at P. lilacinum diversity in this project. All groups will also have access to 3 PCR primers for Sanger sequencing: one to characterise fungi to genus (ITS) and 2 others to take a deep dive on the identity of P. lilacinum morphotypes. For reference see Naimul’s work on Metarhizium. (https://eprints.qut.edu.au/view/person/Islam,_Shah.html) and https://doi.org/10.1016/j.funeco.2022.101179.
Soil Ecologists: The isolation of facultative root endophytes, particularly P. lilacinum, the effects of burning on abundance and diversity, and diversity within P. lilacinum.
Media Types: Development of media for the production of P. lilacinum variants as an inoculum
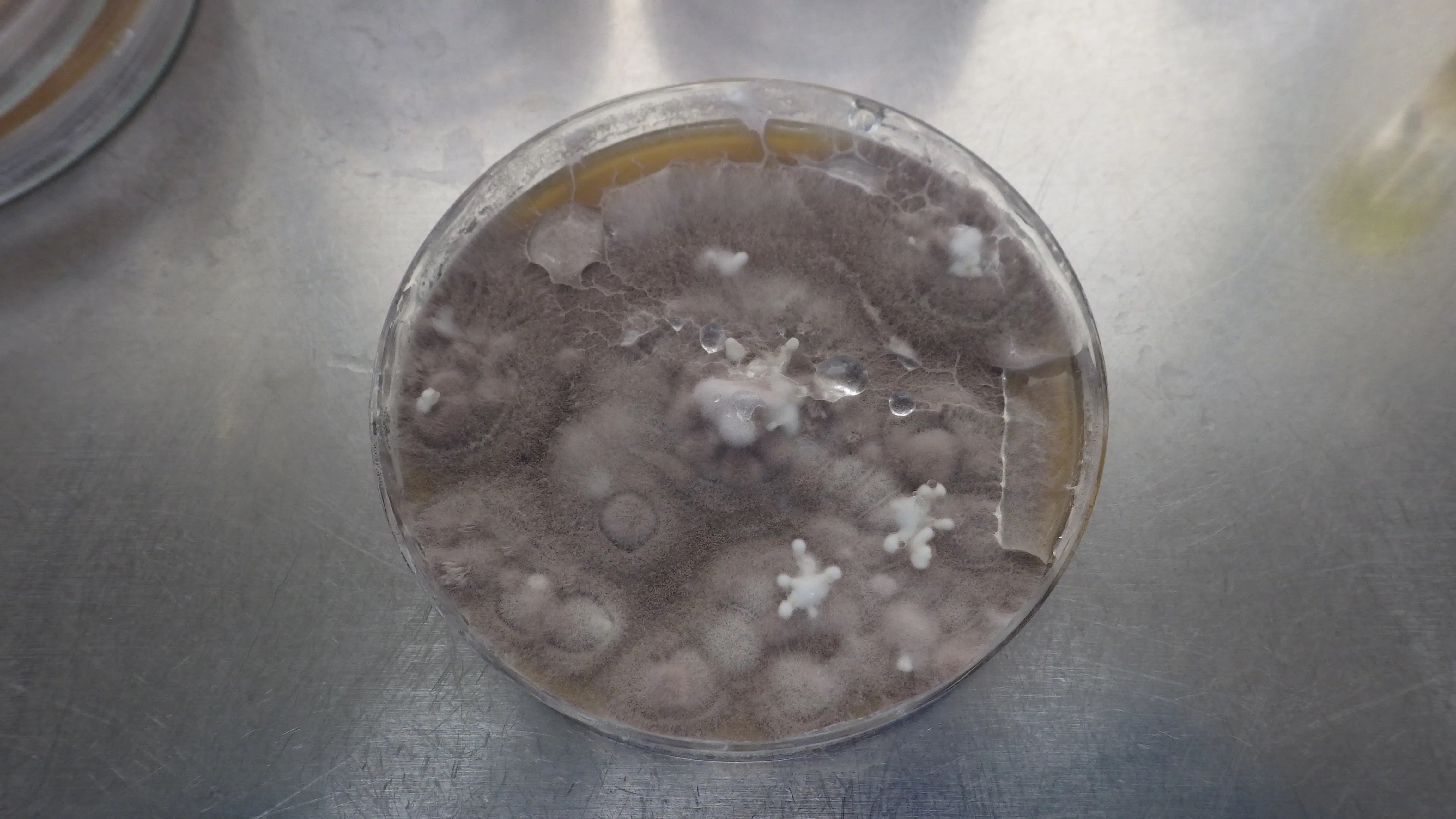
All students (plus artists and the science team) will take part in soil sampling .. at SERF. This will take soil samples at 5 points along a transect across a strip that was burned last year See picture attached). 2 sample points will be outside the burn, and 3 inside it. All will plate out their soil samples onto selective media.
For further background of this process see a report of the 2021 lab run by A/Prof Caroline Hauxwell at Woodford
“